Generic & Specialty Amines
Hydrogen sulfide and carbon dioxide are the principal objectionable acid gas constituents often present in natural gas, synthetic gas, and various refinery gas streams. These acid gas constituents must be removed for corrosion prevention in gas pipelines and process equipment and for health and safety reasons.
The most proven and commercially available technologies for co-capture of H2S and CO2 are by chemical absorption through the use of amine-based solvents. Primary, secondary and tertiary amines are the most common chemical solvents in the gas sweetening industry.
Monoethanolamine (MEA) and Diglycolamine as primary, Diethanolamine (DEA) as secondary and Methyl diethanolamine (MDEA) as tertiary amines are four common chemical absorbents used in refineries to remove acid gases. Low vapor pressure, energy requirements, corrosivity, evaporation losses and high capacity and stability are favorable advantages of these solvents.
Blended and Formulated amine solvents combine the higher CO2 reaction rates of the secondary amine with the higher CO2 loading capacity of the tertiary amine, which may lead to a significant lower solvent circulation rates, compared to a single amine solvent.
Amine sweetening
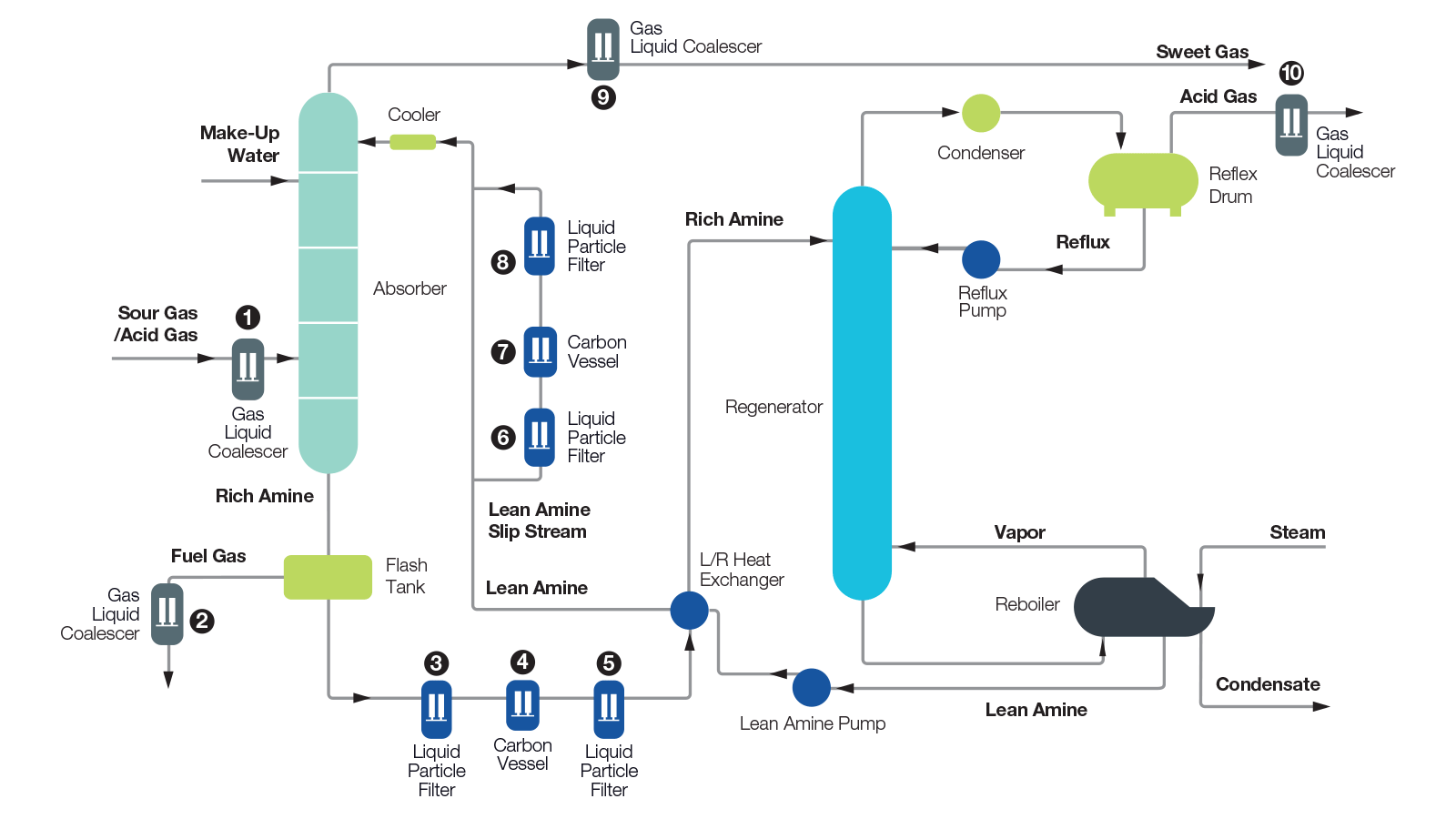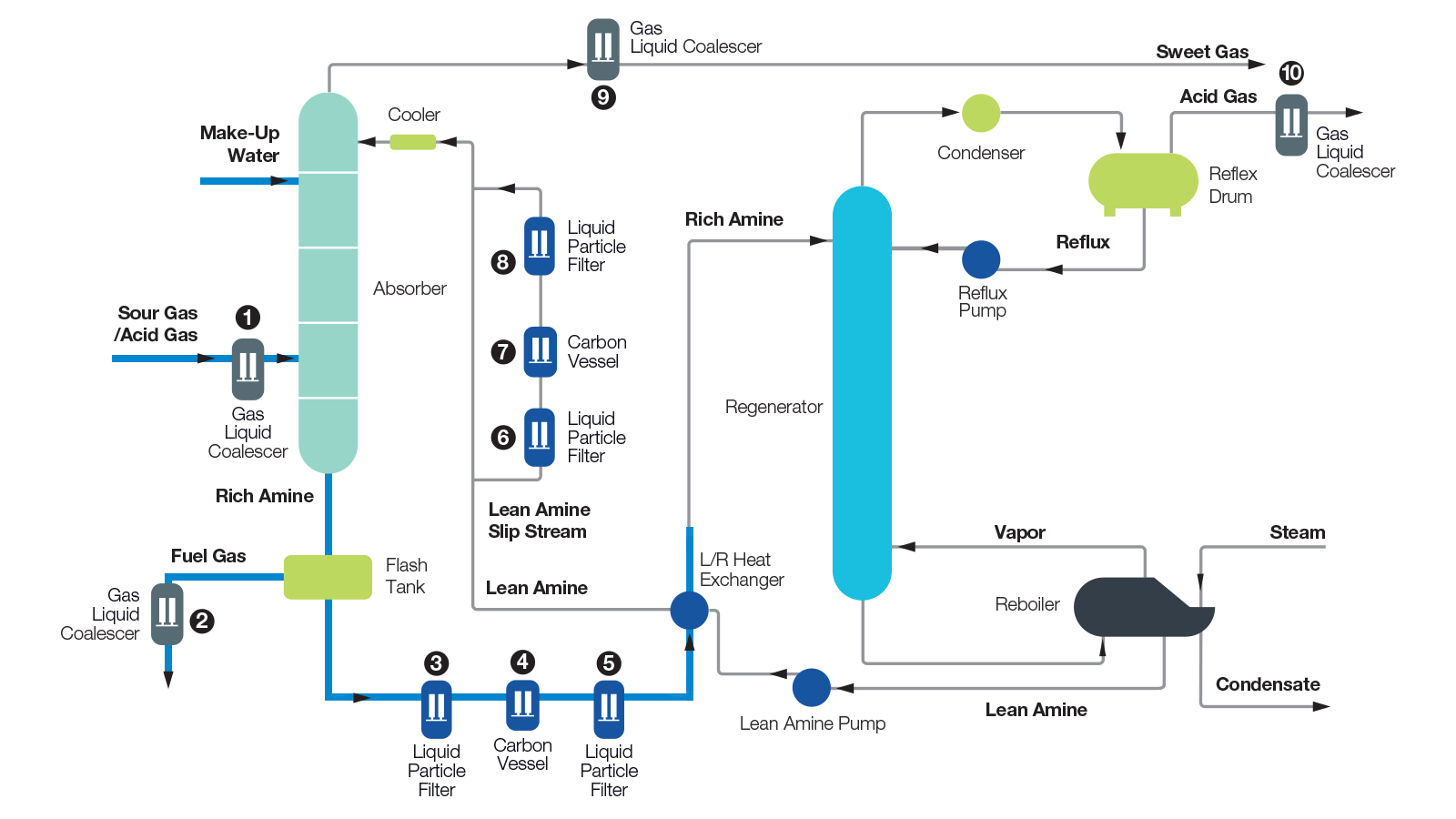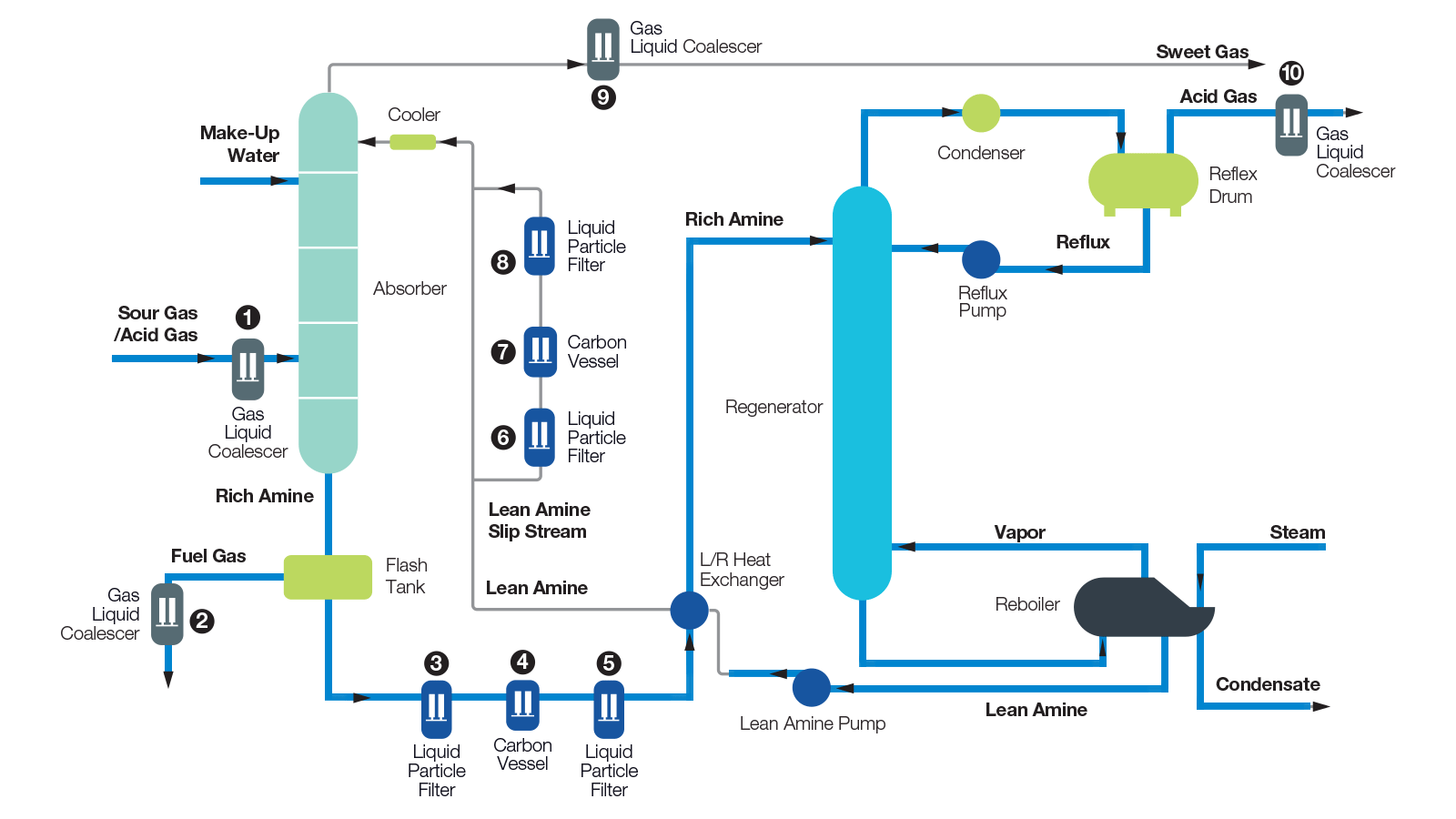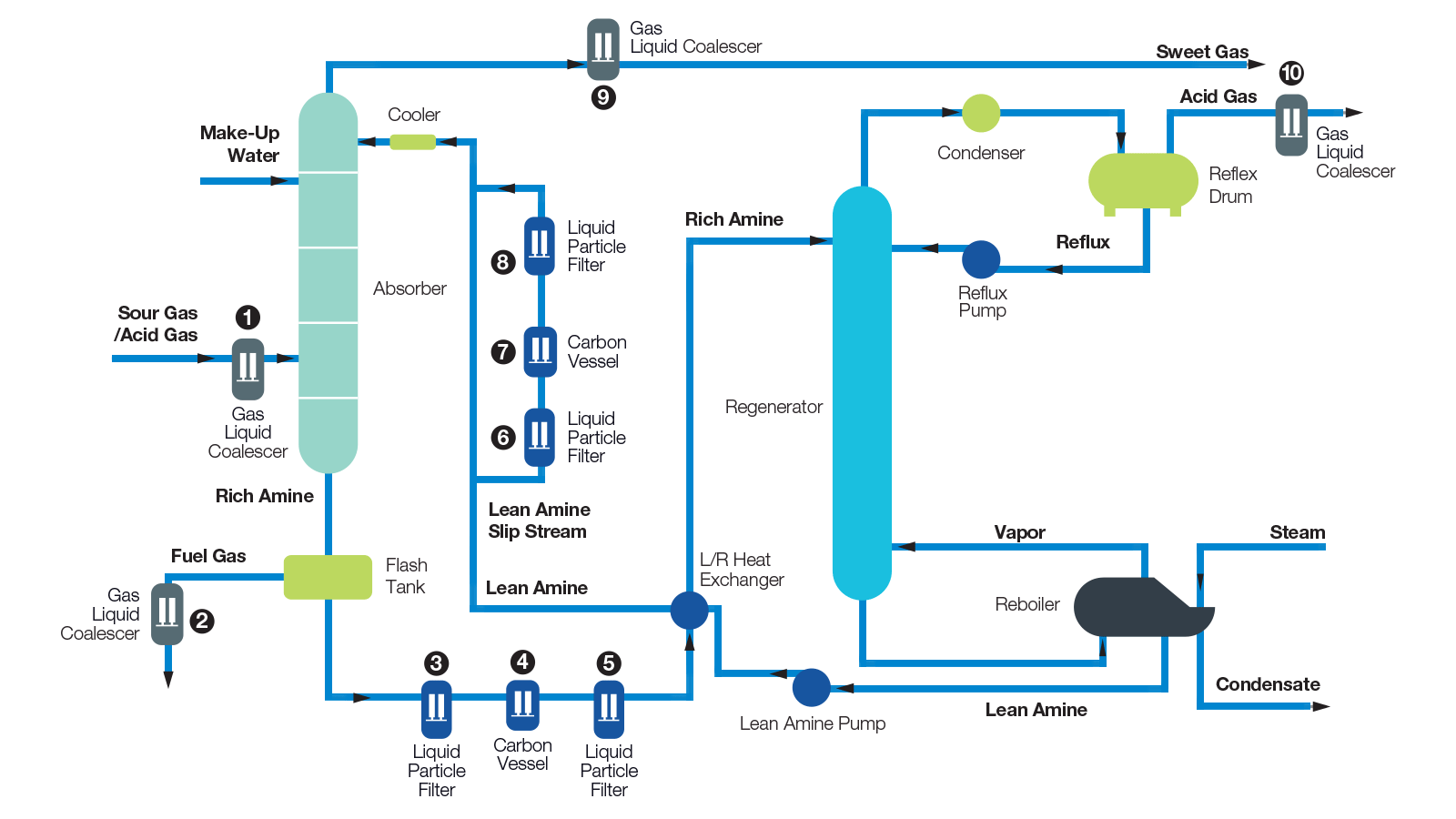
GP Gaspack offers a wide range of amines to satisfy customer requirements:
- Monoethanolamine (MEA)
- Diglycolamine (DGA)
- Diethanolamine (DEA)
- Metil Diethanolamine (MDEA)
- Specialties Amines
Monoethanolamine (MEA)
MEA is a primary amine and is the most aggressive amine among the group. MEA is also the lowest cost solvent. MEA can remove both H2S and CO2 from gas stream to meet sales gas specifications. MEA solvent is very corrosive at high acid gas loadings and high solutions concentrations. It reacts irreversibly with carbonyl sulfide (COS) and carbon disulfide (CS2), which can degrade the solvent and losses and form heat stables salts. MEA has a higher vapor pressure than other amines. To maintain the amine activity, thermal reclaiming of a slipstream of the circulating amine is required.
Diglycolamine (DGA)
As a primary amine, DGA is similar in many respects to MEA except that it exhibits lower vapor pressure, lower solution corrosion tendency, improved solution properties and can operate at higher concentrations than MEA. The higher operating concentration (up to 60% wt%) results in significantly lower circulation rates and energy consumption.
DGA can remove COS, CS2, and partially mercaptan but also form degradation products which are not reversible at normal amine regenerator temperature. Degradation reaction produce N, N´, bis (hidroxyethoxyethyl) urea (BHEEU) an N, N´, bis-(hidroxyethoxyethyl) thiourea (BHEETU). To maintain the amine activity, thermal reclaiming of a slipstream of the circulating amine is required.
Diethanolamine (DEA)
DEA is a secondary amine. The amine reactivity and corrosivity is lower than primary amines. Vapor pressure and heat of reaction are also lower. DEA is a common amine used for H2S and CO2 removal in refineries due to its stability with contaminants in the refinery gas streams. DEA can partially remove COS and CS2 however the reaction rate of DEA with COS and CS2 is lower than with MEA.
Methyl Diethanolamine (MDEA)
MDEA is tertiary amine, is the most widely used gas treating agent today. Unlike primary and secondary amines, MDEA cannot react with CO2 by carbamate reaction. It can only absorb CO2 by the slow bicarbonate formation. This property allows MDEA to selectively remove H2S when treating a gas stream containing both H2S and CO2. By reducing CO2 absorption more solvent is available for H2S removal. Other advantages of MDEA includes low vapor pressure and solution losses, low energy for solvent regeneration, low corrosiveness, and resistance for degradation.
Specialty Amines
To meet stringent emission requirements, and to take advantage of the low energy consumption of MDEA, MDEA can be blended with chemicals promoters to meet the treating requirements. Promoters work by a shuttle mechanism and affect thermodynamics, but more importantly, they can control the reactivity with CO2. Varying the concentration of chemicals activators as piperazine offers one of the solutions to meet the acid gas specifications in H2S and CO2. With other proprietary additives, they can be used for removal of mercaptans and COS and other contaminants.
Applications |
|
Tail Gas Treatment |
The tail gas treatment process reduces sulfur vapor and SO2 contained in the tail gas from the Claus process to H2S and absorbs it in the absorbing solution (amine), and returns it to the Claus process, thereby achieving a high rate of sulfur recovery. |
Syngas Treatment |
Organic feedstocks can be converted into synthetic gas (syngas) through a variety of processes, including steam reforming and partial oxidation. The main components of syngas are hydrogen and carbon monoxide. Syngas is a very versatile gas because it can be converted into high-value streams such as chemicals, pure hydrogen, liquid fuels, or power. |
Refinery Gas Treatment |
Refineries are designed to process crude oil streams into a wide variety of valuable products including gasoline, diesel, kerosene, LPG, waxes, and more. Most crude oil streams are sour, containing a percentage of organic sulfur species that are not tolerable in the product streams. The most effective process for removal and disposal of the sulfur components is conversion to hydrogen sulfide (H2S) and subsequently elemental sulfur via the Claus process. |
Natural Gas Processing |
Natural gas extracted from the earth may contain, in addition to valuable hydrocarbon components, undesirable contaminants such as water, mercury, carbon dioxide (CO2), hydrogen sulfide (H2S), mercaptans, and carbonyl sulfide (COS). CO2, H2S, mercaptans, and COS are generally termed acid gases because they form acids in aqueous solutions. These contaminants need to be removed, to varying degrees, according to the target use for the gas e.g., LNG production, pipeline transportation, or local consumption for power production. In addition, some gas wells contain sulfur species that also need to be removed. |
Ammonia Production |
In natural gas-based ammonia production, steam-reforming and partial-oxidation technology are used to convert natural gas into syngas consisting mainly of hydrogen and nitrogen. The carbon present in the natural gas feedstock is mainly converted to CO and CO2. The syngas is then processed in a shift reactor to convert CO into CO2, which generates additional hydrogen. Complete removal of CO and CO2 is required as they both are poisons for the ammonia synthesis catalyst. |
Acid Gas Enrichment |
Processes for enriching acid gases for Sulphur plant feeds and for producing a commercially valuable CO2 by-product include a sour gas stream contacting an absorbent in an absorber and regenerating the absorbent to produce a regenerated absorbent and an acid gas stream. A portion of the acid gas stream is recycled to produce higher ratios of hydrogen sulfide to carbon dioxide. Multiple absorbers and recycle streams can be used. |